

Published online 2014 Jun 11
Francesco Saverio Martelli, Marialaura Martelli, Claudio Rosati, and Elena Fanti Author information Copyright and License information DisclaimerSUMMARY
Objective
The potential role of VDR gene variations in modulating periodontal susceptibility have been a subject of scientific investigations. The aim of this paper is to perform a literature review of the potential correlation between Vitamin D Receptor (VDR) gene polymorphisms and periodontal disease.Materials and Methods
A PubMed literature search was made using “vitamin d receptor polymorphisms periodontal disease“ as keys words. Only clinical studies in “Humans” as species and “English” as language were considered. Titles and abstracts of all identified records were examined to determine if the candidate articles contained sufficient information on the association of the VDR polymorphisms and the risk of development periodontal disease.Conclusions
Vitamin D may affect the risk of developing periodontal disease via an effect on bone mineral density or via immunomodulatory effects. There are scientific evidences about the correlation between some VDR polymorphisms, periodontitis and bone metabolism. The use of new simple and economics diagnostic techniques of genetic screening, allows to the dental specialists to identify periodontal patients with possible decreased bone mineral density. The complete acquisition of awareness by dentists of the strong relationship between skeletal bone density with periodontal health and osteointegrated implant success, could open a new therapeutic approach for periodontists with an important role in the early detection of osteoporosis and a better patient compliance of the periodontal therapy.
Keywords: periodontitis, vitamin D receptor polymorphisms, vitamin D, periodontal disease, genetic susceptibility periodontitisIntroduction
Vitamin D is a well known regulator in musculoskeletal health through the mediation of calcium absorption and mineral homeostasis. Evidence has demonstrated that vitamin D deficiency may place subjects at risk not only for low mineral bone density but also in other metabolic pathways such as those involved in immune response, chronic inflammatory diseases and cancer (1). However, there are no studies demonstrating the role that vitamin D deficiency can get in the etiopathogenesis of oral disease such as periodontitis. Moreover, dentists have not yet well understood the importance of a good bone metabolism for a successful dental practice in bone regeneration procedures and osseointegration of implants. An insufficient bone base which cannot guarantee the necessary stability of the implants placed in it, has been one of the biggest problems of implantology in the past.
Periodontitis is a multifactorial inflammatory disease of the periodontum that represents an increasing serious health problem in the worldwide population so that the World Health Organization has included this pathology among its primary prevention programs (2). The relationship between periodontitis and systemic diseases has increasingly recognized due to the fact that the periodontal pathogens might affect distant sites and organs, and thus have an effects on overall health. A large number of epidemiological studies have recently linked poor oral health with cardiovascular diseases, diabetes, low birth weight, preterm births, rheumatoid arthritis etc. (3–6). The microbiological shift of the microbial communities found in gingival sulcus from predominantly aerobic, gram-positive and commensal oral bacterial species to predominantly gram-negative and anaerobic bacterial species, is essential to the development of periodontitis. However, the individual susceptibility to periodontal disease is strongly influenced by host immune response. Scientific evidences support the concept of periodontal infectogenomics which highlights how the host genetic profile modulates the composition of the subgingival microbiota, affecting persistence of pathogenic bacteria and therefore increasing the risk for the development of periodontitis. These studies emphasize the importance of the assessment of genetic profile in periodontal patients, in order to develop a personalized periodontal treatment plan (7). It is precisely because of its effects on bone metabolism, that Vitamin D could have a central role in dental practice. This review focuses on scientific evidences for a potential correlation between Vitamin D Receptor (VDR) polymorphisms and periodontal disease.

Vitamin D physiology
Vitamin D3 (cholecalciferol) is a secosteroid produced in the skin by a photochemical reaction of 7-dehydrocholesterol. This initial vitamin D compound is inactive and it is then hydroxylated at the 25-position in the liver by the 25-hydroxylase hepatic, to form 25 OH vitamin D3 (25-hydroxycholecalciferol), an inactive compound as well, but the most reliable measurement of an individual’s vitamin D status. The 25OH vitamin D3 is further hydroxylated in the kidney to the active compound 1,25 dihydroxyvitamin D3 (1,25(OH)2 D3 or calcitriol). 1,25(OH)2D3 acts on the intestine, where it stimulates calcium re-absorption, and upon bone, where it promotes osteoblast differentiation and matrix calcification (8).Vitamin D receptor polymorphisms
The biologically most active metabolite of vitamin D 1,25(OH)2D3 (calcitriol) acts by binding an intracellular receptor Vitamin D Receptor (VDR), which is present in a wide variety of tissues. VDR belongs to a superfamily of transacting transcriptional regulatory factor, such as for example the steroid and thyroid hormone receptors. The gene encoding the VDR is located on the chromosome 12 (12q13.11) and encompasses two promoter regions, eight protein-coding exons and six untranslated exons (9). The major steps involved in the control of gene transcription by VDR include ligand binding, heterodimerization with retinoid X receptor, binding of the heterodimer to vitamin D response elements, and recruitment of other nuclear proteins into the transcriptional pre-initiation complex. The ensemble of protein-protein-DNA interactions promotes transcriptional initiation of a battery of target genes, leading to the pleiotrophic effects of the 1,25(OH)2D3 hormone (10). Researchers have identified over 200 genes directly influenced by vitamin D and 2776 binding sites for the vitamin D receptor along the length of the genome. These were unusually concentrated near a number of genes associated with susceptibility to autoimmune conditions such as Crohn’s disease, lupus and rheumatoid arthritis (11).
It can therefore be supposed that VDR gene polymorphisms and alterations on the VDR mediated signaling pathways could lead to different important cellular effects on gene activation such as enhanced/reduced transcription, calcium metabolism, cell proliferation and immunological response.
VDR gene contains 4 polymorphic regions, three of which located at the 3′-end and could be detected by restriction fragment length polymorphisms (RFLPs) using the restriction enzymes BsmI, ApaI (intron 8) and TaqI (exon 9), while the fourth is detected by restriction enzyme FokI in the start codon (12, 13).
FokI polymorphism creates a start codon in exon 2 resulting in an alternative start site due to thymine (T) to cytosine (C) substitution in the first translation initiation codon (ATG to ACG), which generates long and short variants of VDR. Individuals with the T allele (designated f) initiate translation at the first ATG site, giving rise to a full length VDR protein (427 amino acids). Conversely, individuals with the C allele (designated F) initiate translation at the second ATG site instead of the first, resulting in a truncated protein with three amino acids less. This is the only known VDR polymorphism resulting in two different VDR protein products (14).
The Apal and the Bsml polymorphisms of the VDR gene are considered to be silent single nucleotide polymorphisms (SNPs). These polymorphisms (G/T and A/G respectively) do not change the amino acid sequence, the amount or function of the VDR encoded protein. However, they may affect gene expression through regulation of mRNA stability (15, 16).
The VDR TaqI (-1056) is characterized by a single base transition (T < C) leading to a synonymous change at codon 352 in exon 9 of the VDR gene that creates a TaqI restriction site. The resulting alleles are called “t” (TaqI site present) and “T” (TaqI site absent). The allele “t” correlates with increased transcriptional activity, mRNA stability, and high serum level of 1,25-D3 (17, 18). However, the mechanisms by which the BsmI, TaqI, and ApaI polymorphisms affect VDR function are still unclear.
The CDX2 polymorphism is located in the promoter region of the VDR gene in exon 1, more exactly in a binding site for the Cdx2, an intestinal specific transcription factor that has been shown to activate VDR gene transcription in the intestinal tract (19). This polymorphism consists in a G to A substitution and results in a more active and strongly binding of Cdx2 transcription factor in case of allele A, with greater transcriptional activity respect to the G allele. As consequence, the A allele may induce an higher VDR expression in the intestine and therefore an increased bone mineral density (BMD) through a better intestinal absorption of calcium (16, 20, 21).
Several studies have shown the presence of linkage disequilibrium across the VDR gene. Linkage disequilibrium can be defined as the co-occurrence of alleles of adjacent polymorphisms with each other, so the presence of a polymorphism allows us to predict what it is linked to. With reference to VDR gene there is concordance of a strong linkage disequilibrium between BsmI, TaqI and ApaI while FokI polymorphisms don’t appear to be in linkage disequilibrium with any other VDR polymorphisms and can be considered as an independent marker in the VDR gene (16, 22). Even though the great number of studies that have emphasized the association with VDR polymorphisms and various diseases, the real function of these VDR alleles on gene transcription, are not yet fully well understood, thus suggesting that these polymorphisms may occur in linkage disequilibrium with other functional polymorphisms in the VDR gene. Association studies have underlined the correlations of these VDR polymorphisms with autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, type I diabetes mellitus, but also cancer, increased susceptibility to microbial infection (periodontal disease) and obviously diseases related to disorders of bone metabolism (osteoporosis). It should be noted, as in all studies of genetic variation, that the results produced in literature could be affected by ethnic variations in the occurrence of VDR polymorphisms.
VDR polymorphisms and periodontal disease: a review of literatureA PubMed (National Center for Biotechnology, National Library of Medicine) literature search was made using “vitamin d receptor polymorphisms periodontal disease“ as keys words with additional activated filters “Humans” as species and “English” as language. Titles and abstracts of all identified records were examined to determine if the candidate articles contained sufficient information on the association of the VDR polymorphisms and the risk of development periodontal disease.
TaqIMost of the articles focused on the analysis of VDR TaqI polymorphism in order to evaluate the potential association of this VDR gene polymorphism to an increased individual susceptibility in developing periodontitis associated with alveolar bone loss. In particular, the TT genotype and the presence of T allele are associated with chronic periodontal disease in Japanese, Chinese and Caucasian subjects (23–27). This data is also in accordance with our previous study which showed a strong correlation between TT genotype of Italian subjects and chronic periodontitis (OR 3.59) but also with aggressive periodontal disease (28). In a cross-sectional study, Borges and co-authors examine the relationship between TaqI polymorphism and subgingival microbiota in Brazilian adults with chronic periodontitis, showing an association of Tt genotype with the disease without observing any association between genotype and bacterial component (29). The VDR TT genotype has been correlated with lower serum levels of Vitamin D3 (17) and with a lower bone mineral density value in postmenopausal woman predisposed to a major risk of osteoporosis (30). Low serum levels of Vitamin D3 due to reduced activation of the provitamin D by UV radiation, to VDR polymorphisms or to nutritional factors, are associated with increased circulating-reactive protein (31), with cardiovascular disease (32) and with autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis and multiple sclerosis (33). Furthermore, Vitamin D deficiency induces a decreased bone mineral density at skeletal level including maxilla and mandible, with an increased alveolar porosity and more rapid alveolar bone re-absorption following invasion by periodontal pathogens. These evidences may explain a higher susceptibility to periodontitis of patients showing VDR TT genotype, hypothesizing a more difficult host response to periodontopathogenic bacteria and to a marked bone loss. On the contrary, the studies of Hennig and Sun found an association between the carriage of the less-frequent VDR t allele with an increased risk of developing early-onset periodontal disease in Caucasian and Chinese subjects respectively (34, 35)

By comparing periodontal disease progression and ApaI VDR polymorphism in adult men, Inagaki et al. demonstrated an association between AA genotype and the highest rates of progression of alveolar bone loss and tooth loss compared to Aa or aa genotypes (36). Moreover, Naito et al. found that AA genotype correlates with an increased risk of severe chronic periodontitis (37) among Japanese males.
BsmIPrevious studies conducted on healthy subjects and periodontal patients in Japanese and Brazilian population, agree in rejecting the hypothesis of the correlation between the only BsmI polymorphism and periodontal disease since no statistical differences in distribution of BsmI VDR polymorphism are founded between the two groups. Vice-versa, in combination with Fc-gammaRIIIB genotype, BsmI may be associated with generalized early-onset periodontitis or, as demonstrated by de Brito, when the allele B of BsmI polymorphism and the T allele of TaqI polymorphism were present, the TB haplotype seemed to increase susceptibility to chronic periodontitis (37–39). The findings of Gunes indicates that the ApaI, BsmI and TaqI polymorphisms analyzed individually, did not show statistically significant differences in their frequencies in a Turkish population of healthy subjects and patients affected by chronic periodontal disease while, also in this case, the presence of allele T (TaqI) and B (BsmI) in the haplotype BT is over-represented among chronic periodontal cases (40).
FokIPark et al. found a correlation between the ff genotype of the FokI VDR polymorphism, with a greater individual susceptibility to developing generalized aggressive periodontal disease. This data was confirmed by the study of Naito and could be explained by the fact that ff genotype seems to be responsible for an increased bone re-absorption and an enhanced inflammatory response (37, 41). On the contrary, the study of Li and co-authors highlights as the Chinese patients showing the F allele have an increased risk of developing generalized aggressive periodontitis (42).
CDX2To our knowledge there have not been yet studies of correlation between CDX2 polymorphisms and periodontal disease.
Vitamin D and periodontal disease
1,25(OH)2D3 plays a role in maintaining oral health through its effects on bone and mineral metabolism and its anti-inflammatory and immunomodulatory properties (43). Since alveolar bone re-absorption is a major characteristic of periodontal disease, it is possible to speculate that mediators of bone metabolism like VDR and its genetic polymorphisms play a role in determining the individual susceptibility to developing periodontitis. For these reasons VDR gene could be considered an interesting candidate gene usable for screening in periodontal practice, singularly or in combination with other inflammatory markers, and vitamin D supplementation could represent a simple attractive way of regulating bone loss in periodontitis. Moreover, according to the Vitamin D Council, recent estimates indicate than over 50% of the global population risks Vitamin D deficiency across all age groups and such represents one of the most underestimated health problems in the world (44, 45). In addition to genetic factors, about 80% of Vitamin D levels in humans are prevalently influenced by exposure to sunlight and approximately 15% by dietary intake. Vitamin D status varies according to latitude, season, skin pigmentation, work occupation (outdoor or indoor), age (elderly may live mostly indoor) and dietary habits.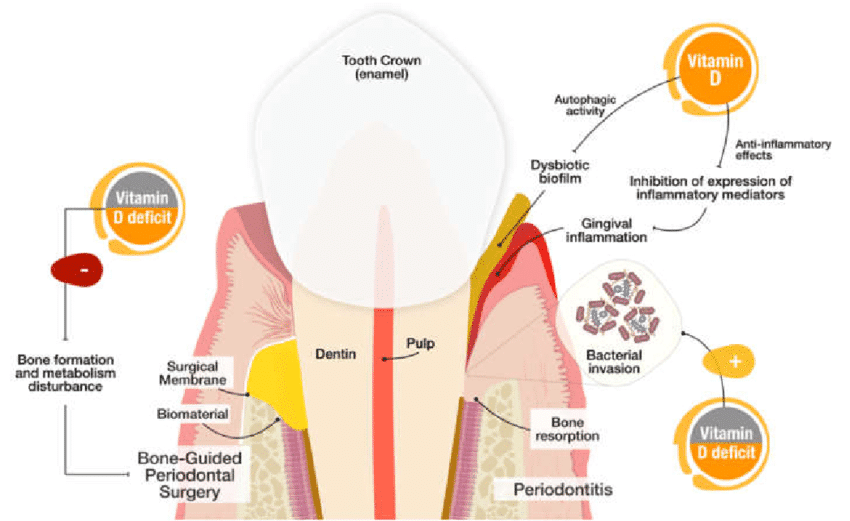
Conclusion
These findings can open a new therapeutic approach for periodontists. Dental practice, in fact could have an important role in the early detection of osteoporosis. With the advent of new simple and economics diagnostic techniques of genetic screening, it is possible for the dental specialist to identify periodontal patients with decreased bone mineral density and thus refer the patient to a evaluation of BMD by DEXA. Periodontal patients also diagnosed with osteoporosis/osteopenia should be clinically treated more aggressively to eliminate periodontal pathogens because of the additive risks. Eventually, the dentist could address the patient to an endocrinological specialist to evaluate pharmacological approaches such as ordinary Vitamin D supplements or more specific drugs such as bisphosphonates, calcitonin etc. and lifestyle intervention.
The complete acquisition of awareness by dentists of the strong relationship between skeletal bone density with periodontal health and osteointegrated implant success, also allows patients to better comply to the periodontal therapy, perceiving the periodontists or the dental team as active participants in improving and promoting the general health of patients.
References
1. Khadilkar VV, Khadilkar AV. Use of vitamin d in various disorders. Indian J Pediatr. 2013;80(3):215–218. PubMed> Google Scholar>2. WHO. Strategies and approaches in oral disease prevention and health promotion. 2007. http://www.who.int/oral_health/strategies/cont/en/index.html.3. Nakano K, Nemoto H, Nomura R, et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol. 2009;24(1):64–68. PubMed> Google Scholar>4. Dunning T. Periodontal disease-the overlooked diabetes complication. Nephrol Nurs J. 2009;36(5):489–495. PubMed> Google Scholar>5. Katz J, Chegini N, Shiverick KT, et al. Localization of P gingivalis in preterm delivery placenta. J Dent Res. 2009;88(6):575–578. PMC free article> PubMed> Google Scholar>6. Bingham CO, 3rd, Moni M. Periodontal disease and rheumatoid arthritis: the evidence accumulates for complex pathobiologic interactions. Curr Opin Rheumatol. 2013;25(3):345–353. PMC free article> PubMed> Google Scholar>7. Nibali L, Donos N, Henderson B. Periodontal infectogenomics. J Med Microbiol. 2009;58:1269–1274. PubMed> Google Scholar>8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. PubMed> Google Scholar>9. Baker AR, McDonnell DP, Hughes M, et al. Cloning the expression of full length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA. 1988;85:3294–3298. PMC free article> PubMed> Google Scholar>10. Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–349. PubMed> Google Scholar>11. Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010;20(10):1352–1360. PMC free article> PubMed> Google Scholar>12. Morrison NA, Yeoman R, Kelly PJ, et al. Contribution of trans-acting factor alleles to normal physiological variability: vitamin D receptor gene polymorphism and circulating osteocalcin. Proc Natl Acad Sci USA. 1992;89:6665–6669. PMC free article> PubMed> Google Scholar>13. Hustmyer FG, DeLuca HF, Peacock M. ApaI, BsmI, EcoRV and TaqI polymorphisms at the human vitamin D receptor gene locus in Caucasians, blacks and Asians. Hum Mol Genet. 1993;2:487. PubMed> Google Scholar>14. Whitfield GK, Remus LS, Jurutka PW, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–159. PubMed> Google Scholar>15. Jurutka PW, Whitfield GK, Hsieh JC, et al. Molecular nature of vitamin D receptor and its role in regulation of gene expression. Rev Endocrin Metab Disord. 2001;2:203–216. PubMed> Google Scholar>16. Uitterlinden SG, Fang Y, van Meurs JB, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. PubMed> Google Scholar>17. Morrison NA, Qi JC, Tokita A, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–287. PubMed> Google Scholar>18. Ma J, Stampfer MJ, Gann PH, et al. Vitamin D receptor polymorphisms, circulating vit D metabolites, and risk of prostate cancer in United states physicians. Cancer Epidemiol Biomarkers Prev. 1998;7:385–390. PubMed> Google Scholar>19. Yamamoto H, Miyamot K, Li B, et al. The caudal-related homeodomain Cdx-2 regulates vitamin D receptor gene expression in the small intestine. J Bone Minar Res. 1999;14:240–247. PubMed> Google Scholar>20. Arai H, Miyamoto K, Yoshida M, et al. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding lement in the human vitamin D receptor gene. J Bone Miner Res. 2001;16:1256–1264. PubMed> Google Scholar>21. Fang Y, van Meurs JB, Bergink AP, et al. Cdx-2polymorphism in the promoter region of the human vitamin D receptor gene determines susceptibility to fracture in the elderly. J Bone Miner Res. 2003;18:1632–1641. PubMed> Google Scholar>22. Ingles SA, Haile RW, Henderson BE, et al. Strength of linkage disequilibrium between two vitamin D receptor markers in 5 ethnic groups: implications for association studies. Cancer Epidemiol Biomark Prev. 1997;6:93–98. PubMed> Google Scholar>23. Tachi Y, Shimpuku H, Nosaka Y, et al. Association of vitamin D receptor gene polymorphism with periodontal diseases in Japanese and Chinese. Nucleic Acids Res Suppl. 2001;1:111–112. PubMed> Google Scholar>24. Tachi Y, Shimpuku H, Nosaka Y, et al. Vitamin D receptor gene polymorphism is associated with chronic periodontitis. Life Sci. 2003;73(26):3313–3321. PubMed> Google Scholar>25. Wang C, Zhao H, Xiao L, et al. Association between vitamin D receptor gene polymorphisms and severe chronic periodontitis in a Chinese population. J Periodontol. 2009;80(4):603–608. PubMed> Google Scholar>26. Brett PM, Zygogianni P, Griffiths GS, et al. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res. 2005;84(12):1149–1153. PubMed> Google Scholar>27. Nibali L, Parkar M, D’Aiuto F, et al. Vitamin D receptor polymorphism (-1056 Taq-I) interacts with smoking for the presence and progression of periodontitis. J Clin Periodontol. 2008;35(7):561–567. PubMed> Google Scholar>28. Martelli FS, Mengoni A, Martelli M, et al. VDR TaqI polymorphism is associated with chronic periodontitis in Italian population. Arch Oral Biol. 2011;56(12):1494–1498. PubMed> Google Scholar>29. Borges MA, Figueiredo LC, Brito RB, Jr, et al. Microbiological composition associated with vitamin D receptor gene polymorphism in chronic periodontitis. Braz Oral Res. 2009;23(2):203–208. PubMed> Google Scholar>30. Seremak-Mrozikiewicz A, Drews K, Mrozikiewicz PM, et al. Correlation of vitamin D receptor gene (VDR) polymorphism with osteoporotic changes in Polish postmenopausal women. Neuro Endocrinol Lett. 2009;30(4):540–546. PubMed> Google Scholar>31. Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95(12):787–796. PubMed> Google Scholar>32. Kendrick J, Targher G, Smits G, et al. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–260. PubMed> Google Scholar>33. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med. 2004;229(11):1136–1142. PubMed> Google Scholar>34. Hennig BJ, Parkhill JM, Chapple IL, et al. Association of a vitamin D receptor gene polymorphism with localized early-onset periodontal diseases. J Periodontol. 1999;70(9):1032–1038. PubMed> Google Scholar>35. Sun JL, Meng HX, Cao CF, et al. Relationship between vitamin D receptor gene polymorphism and periodontitis. J Periodontal Res. 2002;37(4):263–267. PubMed> Google Scholar>36. Inagaki K, Krall EA, Fleet JC, et al. Vitamin D receptor alleles, periodontal disease progression, and tooth loss in the VA dental longitudinal study. J Periodontol. 2003;74(2):161–167. PubMed> Google Scholar>37. Naito M, Miyaki K, Naito T, et al. Association between vitamin D receptor gene haplotypes and chronic periodontitis among Japanese men. Int J Med Sci. 2007;4(4):216–222. PMC free article> PubMed> Google Scholar>38. Yoshihara A, Sugita N, Yamamoto K, et al. Analysis of vitamin D and Fcgamma receptor polymorphisms in Japanese patients with generalized early-onset periodontitis. J Dent Res. 2001;80(12):2051–2054. PubMed> Google Scholar>39. de Brito Júnior RB, Scarel-Caminaga RM, Trevilatto PC, et al. Polymorphisms in the vitamin D receptor gene are associated with periodontal disease. J Periodontol. 2004;75(8):1090–1095. PubMed> Google Scholar>40. Gunes S, Sumer AP, Keles GC, et al. Analysis of vitamin D receptor gene polymorphisms in patients with chronic periodontitis. Indian J Med Res. 2008;127(1):58–64. PubMed> Google Scholar>41. Park KS, Nam JH, Choi J. The short vitamin D receptor is associated with increased risk for generalized aggressive periodontitis. J Clin Periodontol. 2006;33(8):524–528. PubMed> Google Scholar>42. Li S, Yang MH, Zeng CA, et al. Association of vitamin D receptor gene polymorphisms in Chinese patients with generalized aggressive periodontitis. J Periodontal Res. 2008;43(3):360–363. PubMed> Google Scholar>43. von Essen MR, Kongsbak M, Schjerling P, et al. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11(4):344–349. PubMed> Google Scholar>44. Says UC. More Than Half the World’s Population Gets Insufficient Amounts of Vitamin DArticles from Clinical Cases in Mineral and Bone Metabolism are provided here courtesy of CIC Edizioni Internazionali